Abivax Announces Late-Breaking Presentation of 8-Week ABTECT Induction Trial Results in Participants With and Without Prior Inadequate Response to Advanced Therapies
Abivax Announces Late-Breaking Presentation of 8-Week ABTECT Induction Trial Results in Participants With and Without Prior Inadequate Response to Advanced Therapies
-
50 mg dose of once-daily obefazimod achieved clinically meaningful improvements, across all endpoints, regardless of prior inadequate response to advanced therapies (AT-IR)
-
50mg dose demonstrated clinically meaningful improvements in clinical remission in participants with and without prior inadequate response to up to 4+ lines of advanced therapies including JAK inhibitors in the pooled ABTECT 1 & 2 Trials (w/AT-IR pbo adjusted D 10%, p=0.0009†; w/o AT-IR pbo adjusted D 22%, p<0.0001†)
-
50mg obefazimod demonstrated clinically meaningful improvement in clinical response in participants with no prior AT-IR (pbo adjusted D 28%, p<0.0001†) and in participants with 4 or more prior AT-IR (pbo adjusted D 29%, p=0.0242†)
-
50mg obefazimod demonstrated clinically meaningful improvements in clinical response in participants with prior failure to JAK inhibitor therapy in the pooled ABTECT 1 & 2 Trials (pbo adjusted D 34%, p=0.0017†)
-
50mg dose demonstrated clinically meaningful improvements in clinical remission in participants with and without prior inadequate response to up to 4+ lines of advanced therapies including JAK inhibitors in the pooled ABTECT 1 & 2 Trials (w/AT-IR pbo adjusted D 10%, p=0.0009†; w/o AT-IR pbo adjusted D 22%, p<0.0001†)
-
25mg and 50mg once-daily obefazimod performed similarly across clinical endpoints in patients who had no prior AT-IR
-
Obefazimod treatment was well tolerated with no new safety signals identified for both the 25mg and 50mg doses
-
Abivax Management to host a conference call today at 9:00 a.m. ET / 3:00 p.m. CET to discuss the results
PARIS, France – October 06, 2025 – 10:00 AM CET – Abivax SA (Euronext Paris: FR0012333284 – ABVX / Nasdaq: ABVX) (“Abivax” or the “Company”), a clinical-stage biotechnology company focused on developing therapeutics that harness the body’s natural regulatory mechanisms to stabilize the immune response in patients with chronic inflammatory diseases, today announced additional clinical data for obefazimod were delivered in a second late-breaking presentation at the United European Gastroenterology (UEG) Meeting in Berlin, Germany. These data, from the Phase 3 ABTECT 8-Week Induction Trials investigating obefazimod for the treatment of moderate-to-severely active ulcerative colitis, highlight additional efficacy endpoint data at week 8 for patients with and without prior advanced therapy inadequate response (AT-IR).
“Despite advances in care, many patients with ulcerative colitis continue to struggle with inadequate disease control and safety concerns that significantly impact their quality of life,” said Silvio Danese, MD, PhD, Professor of Gastroenterology, IRCCS San Raffaele Scientific Institute, and UEG President Elect. "The outstanding results shared today demonstrate meaningful improvements across a spectrum of patients with ulcerative colitis, ranging from those who were naïve to advanced therapies to those who have failed up to 4+ lines of prior advanced therapy, including JAK inhibitors. Taken together with the clinically meaningful improvements across all efficacy endpoints and a continued favorable safety profile, these findings highlight obefazimod's potential in becoming the standard of care for treating a broad spectrum of patients with ulcerative colitis.”
Study Population: A total of 1272 patients were enrolled across the ABTECT trials, with approximately 60% being classified as having an endoscopic subscore of 3. Of the total pooled population, approximately 47% were classified as having a prior inadequate response to advanced therapy (‘AT-IR Yes’) with approximately 21% having failed to respond to a JAK inhibitor. The populations in the pooled ABTECT 1 & 2 trials were generally well balanced.
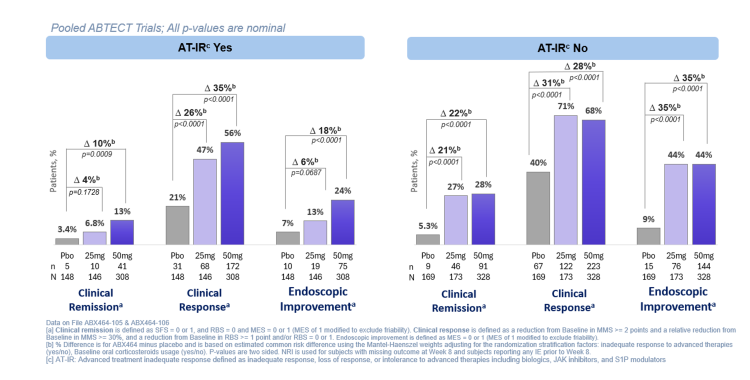
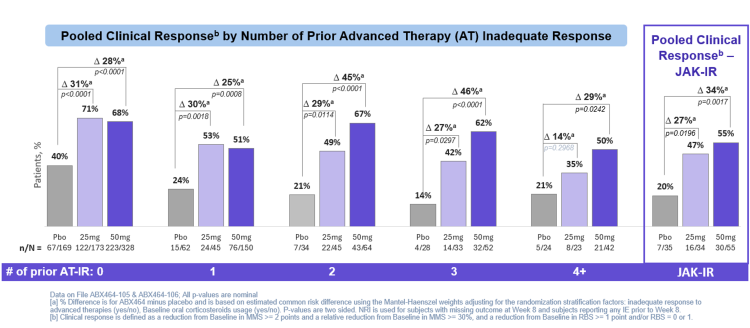
Results: In the pooled ABTECT 1 & 2 trials, treatment with once-daily obefazimod 50mg achieved clinically meaningful improvements in clinical response across all subgroups, including participants with prior AT-IR. In participants without prior AT-IR, obefazimod 50mg delivered a placebo-adjusted difference in clinical response of 28% (p<0.0001), and in participants with up to four or more prior AT-IR, a placebo-adjusted difference of 29% (p=0.0242). Treatment with obefazimod 50mg also demonstrated robust clinical response in participants who had previously failed JAK inhibitor therapy, with a placebo-adjusted difference of 34% (p=0.0017). Treatment with 50mg obefazimod achieved clinically meaningful improvements in endoscopic and histologic endpoints, regardless of prior AT-IR. In addition, for participants without prior AT-IR, the 25mg and 50mg once-daily doses of obefazimod achieved similar efficacy across clinical, endoscopic, and histologic endpoints. Obefazimod continued to be well tolerated with no new safety signals identified.
† ABTECT-1&2 pooled analysis; all p-values are nominal; AT-IR was defined as inadequate response, loss of response, or intolerance to advanced therapies including biologics, JAK inhibitors, and S1P modulators
Investor Conference Call and Webcast
Abivax management will host an investor and analyst conference call today at 9:00 a.m. ET / 3:00 p.m. CET to discuss the topline results. To participate, please use the following dial-in or webcast link:
https://edge.media-server.com/mmc/p/tjj8438w
About Abivax
Abivax is a clinical-stage biotechnology company focused on developing therapeutics that harness the body’s natural regulatory mechanisms to stabilize the immune response in patients with chronic inflammatory diseases. Based in France and the United States, Abivax’s lead drug candidate, obefazimod (ABX464), is in Phase 3 clinical trials for the treatment of moderately to severely active ulcerative colitis.
Contact:
Patrick Malloy
SVP, Investor Relations
Abivax SA
patrick.malloy@abivax.com
+1 847 987 4878
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements, forecasts and estimates, including those relating to the Company’s business. Words such as “anticipate,” “expect,” “potential” and variations of such words and similar expressions are intended to identify forward-looking statements. These forward-looking statements include statements concerning the potential therapeutic benefit of obefazimod. Although Abivax’s management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks, contingencies and uncertainties, many of which are difficult to predict and generally beyond the control of Abivax, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. A description of these risks, contingencies and uncertainties can be found in the documents filed by the Company with the French Autorité des Marchés Financiers pursuant to its legal obligations including its universal registration document (Document d’Enregistrement Universel) and in its Annual Report on Form 20-F filed with the U.S. Securities and Exchange Commission on March 24, 2025 under the caption “Risk Factors.” These risks, contingencies and uncertainties include, among other things, the uncertainties inherent in research and development, future clinical data and analysis, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug candidate, as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, and the availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements. Special consideration should be given to the potential hurdles of clinical and pharmaceutical development, including further assessment by the Company and regulatory agencies and IRBs/ethics committees following the assessment of preclinical, pharmacokinetic, carcinogenicity, toxicity, CMC and clinical data. Furthermore, these forward-looking statements, forecasts and estimates are made only as of the date of this press release. Readers are cautioned not to place undue reliance on these forward-looking statements. Abivax disclaims any obligation to update these forward-looking statements, forecasts or estimates to reflect any subsequent changes that the Company becomes aware of, except as required by law. Information about pharmaceutical products (including products currently in development) that is included in this press release is not intended to constitute an advertisement. This press release is for information purposes only, and the information contained herein does not constitute either an offer to sell or the solicitation of an offer to purchase or subscribe for securities of the Company in any jurisdiction. Similarly, it does not give and should not be treated as giving investment advice. It has no connection with the investment objectives, financial situation or specific needs of any recipient. It should not be regarded by recipients as a substitute for exercise of their own judgment. All opinions expressed herein are subject to change without notice. The distribution of this document may be restricted by law in certain jurisdictions. Persons into whose possession this document comes are required to inform themselves about and to observe any such restrictions.

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
