AtaiBeckley Announces Positive Topline Data from the Phase 2b Open-Label Extension Study of BPL-003, Supporting Safety and Efficacy of a Second Dose in Patients with Treatment-Resistant Depression
-
A 12 mg dose of BPL-003 (mebufotenin benzoate nasal spray) administered eight weeks after an initial 12 mg, 8 mg or 0.3 mg dose in the core study of the Phase 2b clinical trial produced additional rapid, clinically meaningful antidepressant effects that were sustained for up to eight weeks
-
Patients who received an active dose of BPL-003 in the core study of the Phase 2b clinical trial (either 8 mg or 12 mg) met response and remission criteria for depression at increased rates after receiving a 12 mg dose in the OLE study, with 63% in response and 48% in remission at Week 8 in the OLE study (Week 16 of the Phase 2b clinical trial)
-
An End-of-Phase 2 meeting has been scheduled with the U.S. Food and Drug Administration (FDA) to align on clinical trial designs and other aspects of the BPL-003 Phase 3 development program. Anticipate providing guidance on the Phase 3 clinical program in the first quarter of 2026
-
Readout follows announcement that the FDA granted Breakthrough Therapy designation to BPL-003 for treatment-resistant depression
-
Conference call scheduled for 8:00am ET today, November 10, 2025
NEW YORK and AMSTERDAM, Nov. 10, 2025 (GLOBE NEWSWIRE) -- Atai Beckley N.V. (NASDAQ: ATAI) (“AtaiBeckley” or “Company”), a clinical-stage biopharmaceutical company on a mission to transform patient outcomes by developing effective, rapid-acting and convenient mental health treatments, today announced positive topline results from the open-label extension (OLE) study of its Phase 2b clinical trial (NCT05870540) of BPL-003 in patients with treatment-resistant depression (TRD). Findings show that a 12 mg dose of BPL-003 administered eight weeks after a 0.3 mg, 8 mg or 12 mg dose of BPL-003 was generally well-tolerated and provided additional rapid, clinically meaningful antidepressant effects, which were sustained for up to eight weeks.
Srinivas Rao, M.D., Ph.D., Chief Executive Officer and Co-Founder of AtaiBeckley, said, “These new data provide compelling support that redosing with BPL-003 may deliver additional and durable antidepressant effects in patients with treatment-resistant depression while maintaining a favourable safety and tolerability profile. Importantly, two doses, given eight weeks apart, provided antidepressant effects lasting up to four months, further supporting the viability of an intermittent-dose treatment paradigm with a short psychedelic duration, which has the potential to fit within the existing healthcare infrastructure and could minimize the burden on patients and providers. With a significant need for more effective therapies, our priority, following End-of-Phase 2 discussions with the FDA, is to advance BPL-003 into Phase 3 clinical trials with the hope of bringing this promising treatment option to patients as swiftly as possible.”
The Phase 2b clinical trial of BPL-003 was conducted in two parts: an eight-week, quadruple-masked, dose-finding core study designed to evaluate the efficacy and safety of a single 0.3 mg, 8 mg, or 12 mg dose of BPL-003 in patients with TRD, followed by an eight-week OLE study to assess the safety and efficacy of a second 12 mg dose, given eight weeks after the initial dose, regardless of the patient’s Montgomery-Asberg Depression Rating Scale (MADRS) score. Of the 126 patients who completed the blinded core study and were eligible to enroll, 107 continued into the extension study.
Topline efficacy findings:
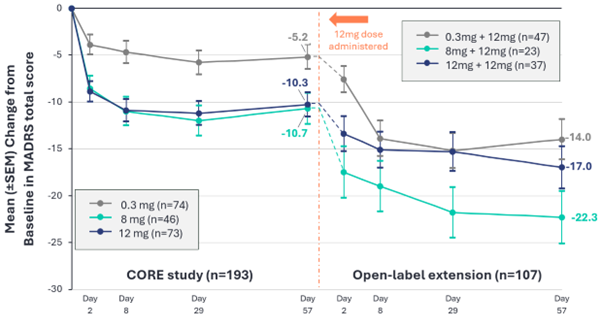
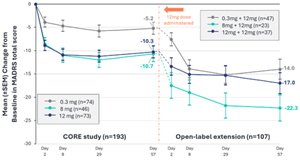
Change from Baseline in MADRS Total Score. Core study efficacy analyses were conducted using a mixed model for repeated measures (MMRM); open-label extension results are based on observed data. OLE study data represents descriptive statistics from subjects enrolled into the open-label extension only.
-
Patients who initially received a 0.3 mg dose of BPL-003 in the core study of the Phase 2b trial (n=47):
- Mean reduction in MADRS score of 14.0 points at Day 57 in the OLE compared to their baseline at the start of the core study, in line with the antidepressant effects seen in patients who received a single active dose in the core study.
-
Patients who initially received an 8 mg dose of BPL-003 in the core study of the Phase 2b trial (n=23):
- Mean reduction in MADRS score of 22.3 points at Day 57 in the OLE (Week 16 of the Phase 2b clinical trial) compared to their baseline at the start of the core study.
- Responder rate (≥50% improvement in MADRS total score) in the OLE was 81% at Day 57 (Week 16 of Phase 2b trial) and remission rate (MADRS score ≤10) was 67% at the same timepoint.
-
Pooled population of patients who received an active dose (either 8 mg or 12mg) of BPL-003 in the core study of the Phase 2b trial (n=60):
- Mean reduction in MADRS score of 19.0 points at Day 57 in the OLE (Week 16 of the Phase 2b clinical trial) compared to their baseline at the start of the core study.
- Responder rate in the OLE was 63% at Day 57 and remission rate was 48% at the same timepoint.
Topline safety findings:
- Safety and tolerability profile was largely consistent with prior studies of BPL-003 and is in line with other previously reported studies of the psychedelic class, showing BPL-003 to be generally well-tolerated. The majority of adverse events occurred on the day of dosing and were classified as mild or moderate in severity and transient in nature.
- Most commonly reported side effects included nausea, headache, administration site pain, administration site discomfort, blood pressure increases and anxiety.
- One serious drug-related adverse event was reported 8 days following administration of the second dose and was resolved with additional in-patient monitoring and support. No other drug-related serious adverse events were reported in the study.
- Average time to meet readiness-for-discharge criteria was within 2 hours of dosing, supporting the potential of BPL-003 to fit within the existing interventional psychiatry treatment paradigm.
Next Steps
The topline results from the OLE study are in line with the previously announced topline results from the eight-week, blinded core study, which demonstrated that both 8 mg and 12 mg single doses of BPL-003 showed statistically significant and clinically meaningful reductions in depressive symptoms when compared to a 0.3 mg low-dose active control for up to eight weeks. Safety and efficacy data from the core and OLE studies of the Phase 2b clinical trial support the selection of the 8 mg dose to advance into Phase 3 clinical development and also support the potential for continued and increased antidepressant effects with repeat dosing. AtaiBeckley is scheduled to meet with the FDA at an End-of-Phase 2 meeting to align on clinical trial designs and other aspects of the Phase 3 development program. The Company anticipates providing guidance on the Phase 3 clinical program in the first quarter of 2026 with Phase 3 clinical trial initiation in the second quarter of 2026, pending the outcome of the FDA meeting.
Conference Call
AtaiBeckley will host a conference call and live webcast today Monday, November 10, 2025, 2025 at 8:00 a.m. ET. The conference call can be accessed by dialling 1-800-715-9871 for participants in the U.S. and 1-646-307-1963 for international callers, with the Conference ID: 1459387. The webcast can be accessed on the Investors section of AtaiBeckley’s corporate website under Events. The presentation and an archived replay of the webcast will be available in the same section of the website for a minimum of 30 days following the event.
About Treatment-Resistant Depression
Depression is a debilitating and life-changing condition affecting nearly 300 million people across the globe. Treatment-resistant depression (TRD) occurs when an individual does not respond to two or more courses of antidepressants and some studies show that it may affect up to 50% of those living with depression, meaning there is a significant unmet need for more effective treatments. TRD is associated with higher rates of comorbid anxiety, sexual dysfunction, cognitive impairment, and reduced quality of life compared to non-resistant forms of depression. The condition places a significant burden on patients, caregivers, and healthcare systems, with elevated healthcare utilization and substantial societal costs.
About BPL-003
BPL-003 is a patent-protected, proprietary intranasal formulation of mebufotenin benzoate, administered via a nasal spray device used in a previously approved drug product. BPL-003 is designed to deliver rapid and durable antidepressant effects from a single dose with a short psychedelic duration, and is being investigated as a potential therapy for treatment-resistant depression (TRD) and for alcohol use disorder (AUD). BPL-003 has been granted Breakthrough Therapy designation by the U.S. Food and Drug Administration and is covered by granted US, UK and European composition-of-matter patents, with multiple further claims pending in various jurisdictions. BPL-003 is an investigational product and has not been approved by the FDA.
About AtaiBeckley N.V.
AtaiBeckley is a clinical-stage biopharmaceutical company on a mission to transform patient outcomes by developing effective, rapid-acting and convenient mental health treatments. It was formed through the strategic combination of atai Life Sciences and Beckley Psytech Limited in November 2025. AtaiBeckley’s pipeline of novel investigational therapies includes BPL-003 (mebufotenin benzoate nasal spray) for treatment-resistant depression (TRD), VLS-01 (buccal film DMT) for TRD and EMP-01 (oral R-MDMA) for social anxiety disorder (SAD), which are in Phase 2 clinical development. It is also advancing a drug discovery program to identify novel, non-hallucinogenic 5-HT2AR agonists for opioid use disorder and TRD. These programs aim to create new breakthroughs in mental health by providing innovative interventional psychiatry therapies that can integrate seamlessly into healthcare systems.
For the latest updates and to learn more about the AtaiBeckley mission, visit www.ataibeckley.com or follow the Company on LinkedIn and on X.
Forward-looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “anticipate,” “initiate,” “could,” “would,” “project,” “plan,” “potentially,” “preliminary,” “likely,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements include express or implied statements relating to, among other things, expectations regarding the potential benefits of the Breakthrough Therapy Designation; progress on and results of Beckley Psytech’s BPL-003 trials; the timing and outcome of development and regulatory review of BPL-003, including the timing of regulatory discussions with respect to Phase 3 trial design for BPL-003; and the potential benefits of BPL-003 for patients with TRD.
Forward-looking statements are neither promises nor guarantees, but involve known and unknown risks and uncertainties that could cause actual results to differ materially from those projected, including, without limitation, the important factors described in the section titled “Risk Factors” in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (“SEC”) and our Proxy Statement on Schedule 14A (the “Proxy Statement”) that was filed with the SEC on September 24, 2025, in each case, as such factors may be updated from time to time in our other filings with the SEC. Atai disclaims any obligation or undertaking to update or revise any forward-looking statements contained herein, other than to the extent required by applicable law.
Contact Information
Investor Contact:
IR@ataibeckley.com
Media Contact:
PR@ataibeckley.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b2d7bd57-a210-4236-bca4-2af76bbd00f7

Chart 1
Change from Baseline in MADRS Total Score. Core study efficacy analyses were conducted using a mixed model for repeated measures (MMRM); open-label extension results are based on observed data. OLE study data represents descriptive statistics from subjects enrolled into the open-label extension only.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.